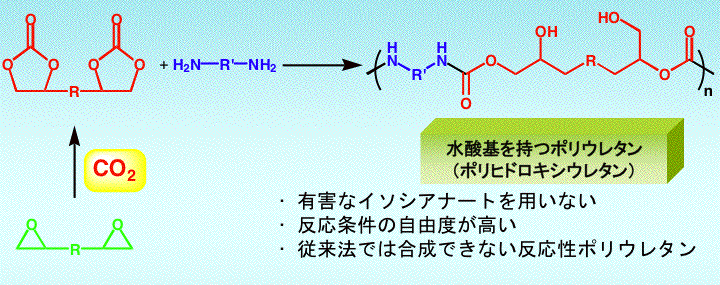
| Functions of polymers bearing five-membered cyclic carbonate moieties |
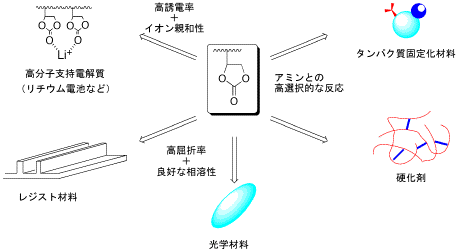 |
Gas-solid phase reactions of gaseous carbon dioxide with solid polymers bearing oxirane |
 Kihara and Endo reported gas-solid phase fixations of carbon dioxide into films of poly(glycidyl methacrylate) containing ammonium salts, although the fixation degree could not be quantitative due to the concomitant anionic cross-linking of the oxirane groups (Kihara & Endo J. Chem. Soc. Chem. Commun. 1994, 937). We optimized the conditions for this gas-solid phase reaction, and attained quantitative incorporation of carbon dioxide. Both soluble and insoluble polymers could be obtained keeping high fixation efficiency (> 95%). |
Concurrent radical polymerization and CO2 fixation for utilizing CO2 by low energy cost |
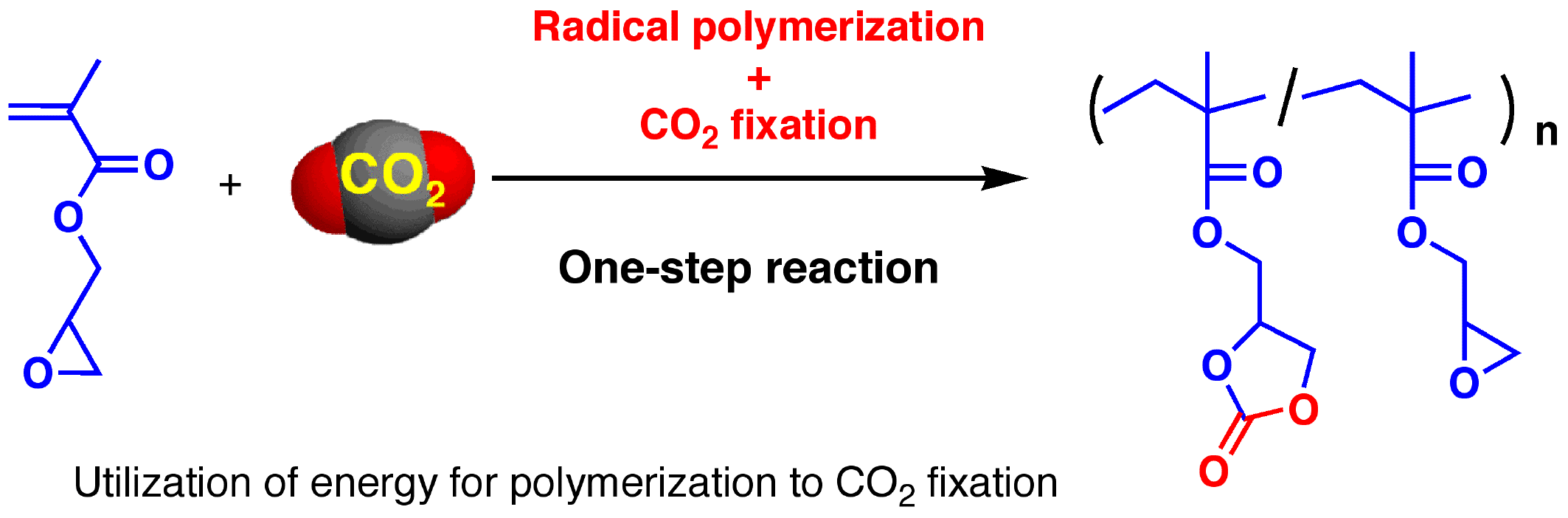 For utilizing carbon dioxide by low energy cost, we developed carbon dioxide proceeding concurrently with other reactions. As depicted in the aboveindicated scheme, carbonate-containing polymers were obtained by radical polymerization of GMA accompanied by carbon dioxide fixation. The unit ratio can be controlled. The energy required for this concurrent reaction is almost competitive with either of the reactions (i.e., radical polymerization of GMA or reaction of carbon dioxide with epoxide), and it indicates that carbon dioxide could be transformed by low energy cost. |