Cyclopolymerization through large ring formation
Large cyclic structures are capable of interacting with low-molecular weight
molecules or ions, which fit to the rings. Such interactions play important
roles in biological activities. Polymers bearing large ring structures
can serve as hosts for supramolecular complexes, and their applications
involve stationary phases for separation, (chiral) templates for reactions,
and ligands. However, large ring formations are typically difficult, and
as a result, polymerizations accompanied by consequtive formations of large
rings are more difficult. Accordingly, we are trying to polymerize monomers
designed for large ring formations.
Synthesis of polymers bearing 11-membered chiral rings
Radical polymerization of a bisacrylamide prepared from a-pinene proceeds
through 11-membered ring formation, and gave the corresponding polymer
in quantitative yields. The MALDI-TOF mass spectroscopy analysis of the
polymer demonstrated that the cyclopolymerization was not accompanied by
undesired inter-polymer propagation.

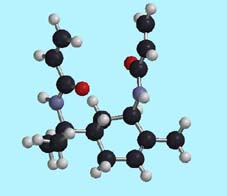
(Optimized geometry of the monomer calculated by the HF3-21G* basis-set
based on the conformation optimized by MMFF calculation)
Potential applications of this polymer are chiral resolution media and
chiral templates。
Nagai, A.; Ochiai, B.; Endo, T. Macromolecules, 2005, 38, 2547-2549.
Controlled cyclopolymerization through 19-membered ring formation
We designed a dimethacrylate whose conformation is fixed by the cyclohexane
ring and the hydrogen-bonding urethane groups. The radical polymerization
of this monomer procceds smoothly, and yields a soluble polymer in quantitative
yield. The RAFT polymerization proceeds via controlled fashion.
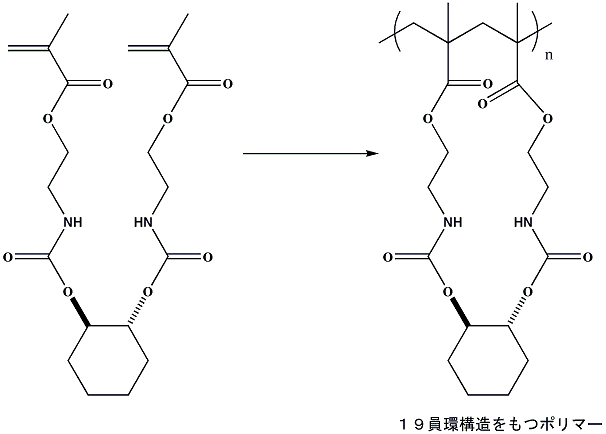
Ochiai, B.; Ootani, Y.; Endo, T. J. Am. Chem. Soc. 2008, 130, 10832-10833.
See also Science Editor's Choice
to research topics


