

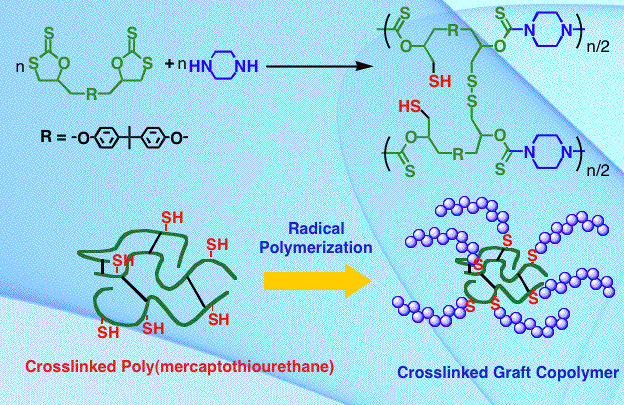
When a trifunctional five-membered cyclic dithiocarbonate was reacted with amines, trithiols bearing functional groups originating from amines were produced. The trithiols are typically stable under air atmosphere. The anionic polymerization of propylene sulfide initiated from the trithiols afforded three-armed polymers bearing functional groups. The trithiols could be converted to tris(trithiocarbonate)s by the reaction with carbon disulfide and benzyl chloride. The tris(trithiocarbonate)s served as RAFT agents for polymerization of styrene.

Three-component polyaddition via transformation of reaction modes from
nucleophilic to radical
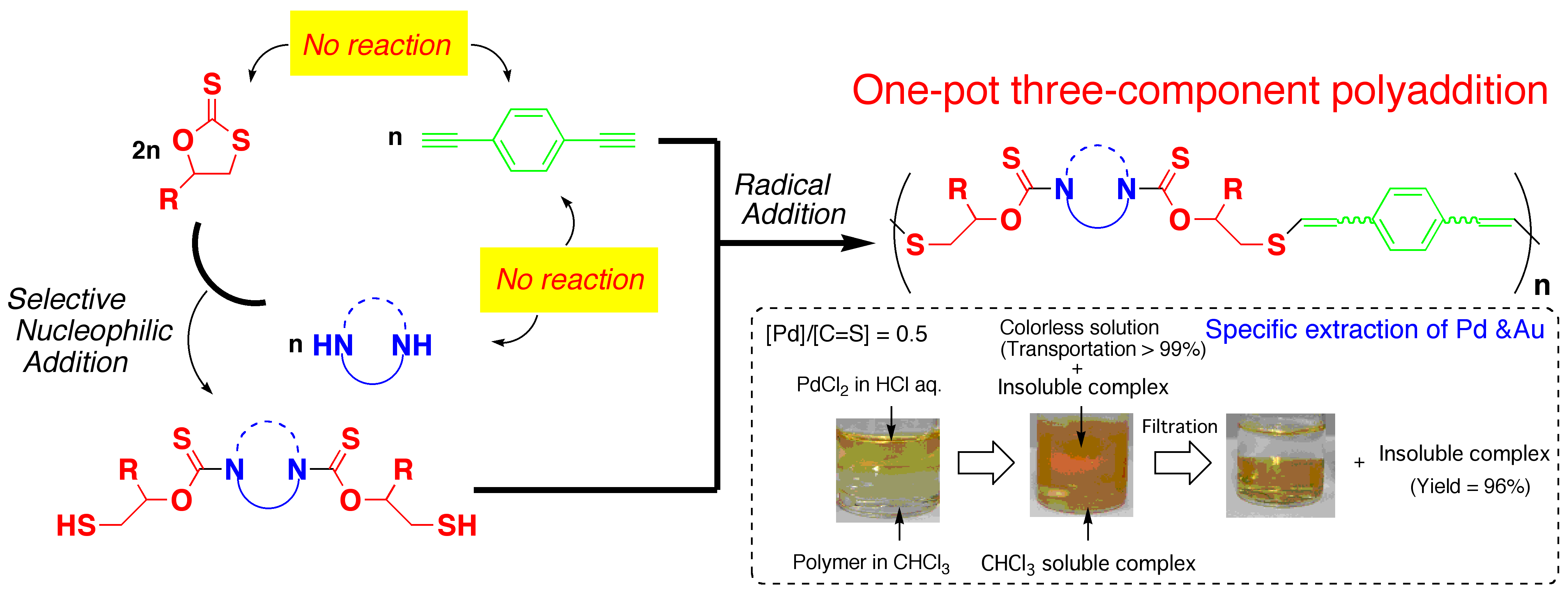
Typical three-component reactions consume two reactive species at first. The products stabler than the initial reactants must react with the remained most unreactive species. Accordingly, excess amounts of components or elimination of stable byproducts, which are responsive to low atom economy, were neccessary. We overcame this problem by transformation of reaction modes. Theused reaction is nucleophilic addition of amines to cyclic dithiocarbonates affording thiols that can react radically. When dithiocarbonates, diamines, and a diyne was used as monomers, the radically reactive diyne never react with others unless thiols are produced by the nucleophilic addition of the amines to the dithiocarbonates. Namely, the polymerization proceeds via the following two steps; the first nucleophilic addition of diamines to cyclic dithiocarbonates, and the subsequent radical addition of the produced dithiols to the diyne. The atom economy is very high (~97%). The resulting polymers can collect Au and Pd salts from their aqueous solutions.
Detail of the works before 2005 is described in the following review.
Carbon Dioxide and Carbon Disulfide as Resources for Functional Polymers.
Ochiai, B.; Endo, T. Prog. Polym. Sci. 30 (2),
183-215 (2005) Link to Science Direct
back to research topics